
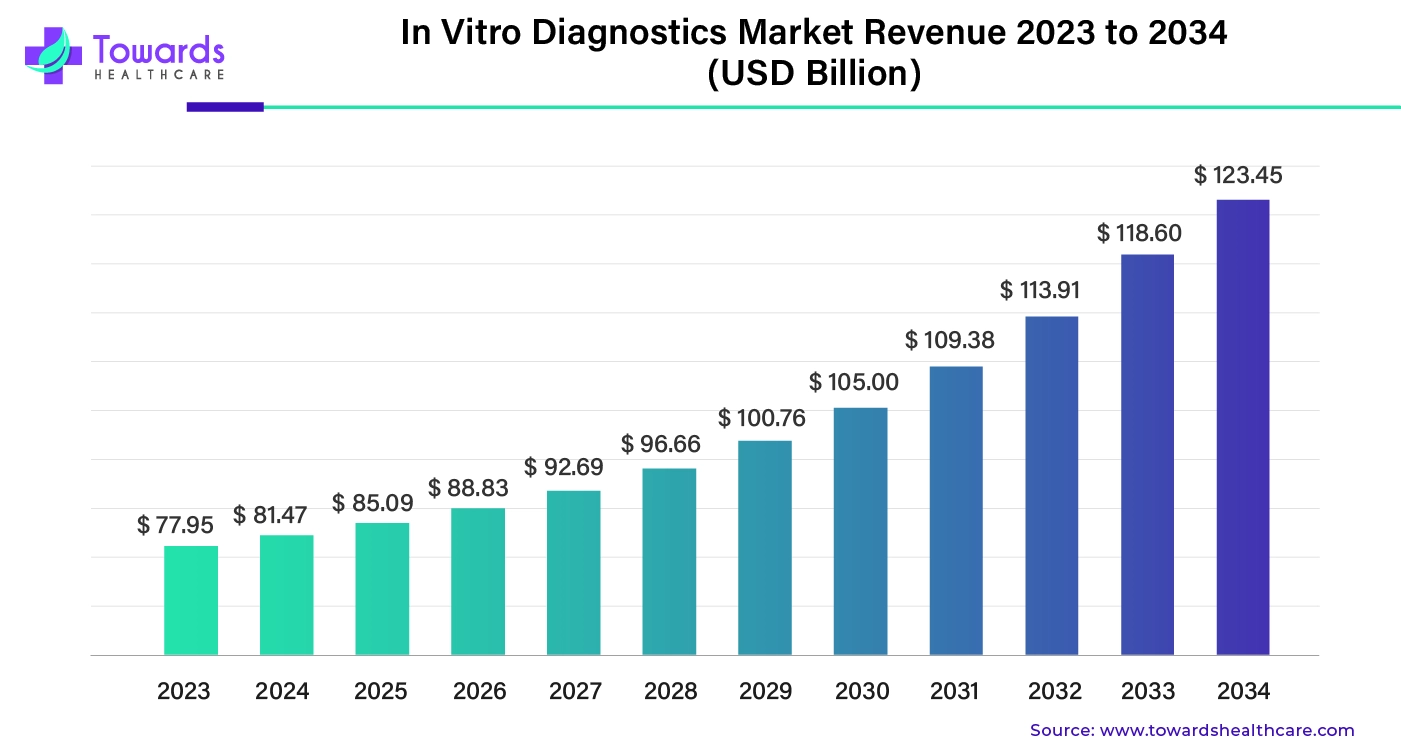
In Vitro Diagnostics Market Size on Track for a USD 123.45 Billion Momentum by 2034, says Healthcare Specialist
According to Towards Healthcare study, the global in vitro diagnostics market size is calculated at USD 85.09 billion in 2025 and is expected to reach around USD 123.45 billion by 2034, growing at a CAGR of 4.45% for the forecasted period.
/EIN News/ -- Ottawa, April 11, 2025 (GLOBE NEWSWIRE) -- The global in vitro diagnostics market size was valued at USD 81.47 billion in 2024 and is predicted to hit around USD 123.45 billion by 2034, a study published by Towards Healthcare a sister firm of Precedence Research.
Get All the Details in Our Solutions – Download Brochure: https://www.towardshealthcare.com/download-brochure/5229
Market Overview
In vitro diagnostics (IVDs) are tests or examinations to detect diseases, conditions, or infections. These tests are performed on human samples such as blood or tissues and can also identify the risk of developing a disease. IVDs detect or measure the biomarkers involved in disease progression. They aid in the early detection of diseases and prevent their spread, improving patient care. This enables healthcare professionals to provide effective treatment through improved clinical decisions. There are generally two types of IVDs, including laboratory-based tests and point-of-care diagnostics.
The rising prevalence of chronic disorders necessitates their early detection, increasing the demand for IVDs. The burgeoning diagnostic and medical device sectors boost the market. The growing demand for personalized medicines also augments market growth. The advent of advanced technologies drives the latest innovations in diagnostic equipment. The increasing investments and collaborations support the development of IVDs.
In Vitro Diagnostics Market Trends
- Rising Prevalence of Chronic Disorders: The rising prevalence of chronic disorders such as cardiovascular disorders, infectious disorders, cancer, and autoimmune disorders. The advent of the COVID-19 pandemic has potentially increased the need for IVDs globally.
- Point-of-Care Diagnostics: Numerous healthcare companies are developing point-of-care diagnostics to detect the presence of disease at the bedside or at home. This benefits the geriatric population and eliminates the need to visit a laboratory for diagnostic tests.
-
Government Policies: Government organizations of various countries launch initiatives for screening and early detection of chronic disorders. This necessitates the use of IVDs for early disease detection and advanced patient care. Government organizations also provide funding to support IVDs' research and manufacturing.
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
Limitations & Challenges of the In Vitro Diagnostics Market
- Regulatory Hurdles: IVDs face regulatory hurdles due to rapidly changing regulatory guidelines. Different countries have different regulations, which makes it compulsory for manufacturers to be compliant with the changing regulations of the country in which the product has to be marketed.
- High Cost: Advanced diagnostics are comparatively more expensive than conventional IVDs. This limits the affordability of numerous healthcare professionals or patients from low- and middle-income countries.
-
Lack of Specificity: Some IVDs have a lack of specificity and sensitivity, as they might give false positive or negative results. This significantly alters the clinical decisions of healthcare professionals and lacks trust among patients, restricting the use of IVDs.
Smart Diagnostics: Future of IVDs
Artificial intelligence (AI) in diagnostics is transforming the future of IVDs by enhancing the diagnostic accuracy and efficiency of testing. AI and machine learning (ML) algorithms can analyze vast amounts of data, including patient records, imaging, and test results. They also enhance the overall speed and precision of detection, potentiating the in vitro diagnostics market growth. They improve healthcare decision-making, reducing the chance for manual errors.
ML enables IVDs to personalize diagnostic tests based on the patient’s genetic profile and medical history. AI and ML can streamline the laboratory workflow and can also interpret the results in real time. The growing demand for remote monitoring and wearable devices potentiate the use of smart diagnostics. Thus, the development of AI-based IVDs is a step toward making a smart healthcare ecosystem using AI, ML, and IoT.
For instance,
-
In September 2024, Ibex Medical Analytics announced the latest advancements in its innovative product platform of cancer diagnostics. These advancements include improved AI algorithms, novel use, enhanced interoperability, and improved user interface.
Elevate your healthcare strategy with Towards Healthcare. Enhance efficiency and drive better outcomes—schedule a call today: https://www.towardshealthcare.com/schedule-meeting
Regional Analysis
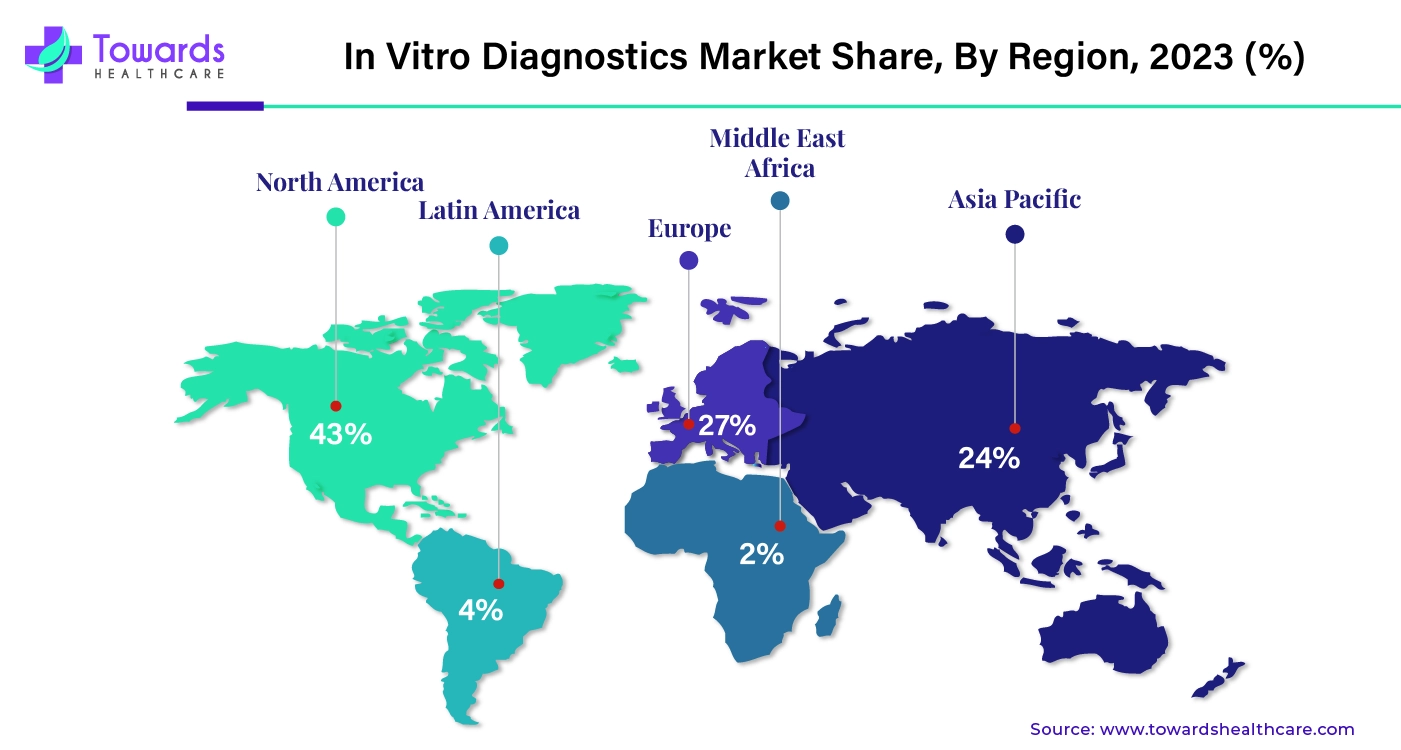
Favorable Regulatory Frameworks Dominated North America
North America dominated the global in vitro diagnostics market in 2024. Favorable government initiatives and regulatory frameworks are the major growth factors of the market in North America. Countries like the U.S. and Canada are at the forefront of developing and distributing IVDs. Advanced healthcare infrastructure and state-of-the-art research and development facilities promote market growth. North America holds the largest share of the pharmaceutical and biotechnology sectors globally, indicating the development and use of IVDs. Moreover, the increasing number of biotech and pharma startups also leads to IVD development.
- United States: The Food and Drug Administration (FDA), the Centers for Devices and Radiological Health (CDRH), and the Centers for Biologics Evaluation and Research (CBER) regulate the approval of IVDs in the U.S. The FDA has approved around 188 companion diagnostic devices, which are IVD types, as of February 2025. The FDA has also approved more than 1,000 AI/ML-enabled medical devices as of March 2025.
- Canada: In February 2025, bioMérieux announced that it received Health Canada approval for its BIOFIRE SPOTFIRE Respiratory/Sore Throat Panel. The panel is a multiplex PCR test to detect and identify nucleic acids from bacterial or viral subtypes.
Get the latest insights on healthcare industry segmentation with our Annual Membership: https://www.towardshealthcare.com/get-an-annual-membership
Favorable Government Support Drive Asia-Pacific
Asia-Pacific is anticipated to grow at the fastest rate in the in vitro diagnostics market during the forecast period. The rising prevalence of chronic disorders due to the increasing geriatric population propels the market. The increasing investments from both government and private institutions promote the development of IVDs. The growing demand for personalized medicines also contributes to market growth. The rising adoption of advanced technologies and growing research and development activities also boost the market. The rapidly expanding medical device sector and growing healthcare infrastructure foster market growth.
- China: The Chinese government actively supports early disease detection for various disorders through screening programs. It aims to have a sweeping action plan for dementia by 2030. The action plan includes prevention, early detection, treatment, rehabilitation, and care of patients with dementia. It expects 15 million people to be trained in dementia care by 2030.
- India: In November 2024, the Indian government launched the Scheme for Strengthening the Medical Device Industry with a total investment of Rs 500 crore. The scheme includes grants of up to Rs 1 crore for clinical performance evaluation of novel in vitro diagnostic products.
-
South Korea: The South Korean government announced an investment of 10 trillion won ($7.6 billion) in the R&D of medical devices between 2023 and 2027. The investment was made to establish Korea as one of the top five medical device export powers. The plan also includes clinical support and quality improvement of IVDs.
Segmental Outlook
Product Insights
The reagents segment held a dominant presence in the in vitro diagnostics market in 2024 and is expected to remain dominant within the time frame. Reagents for IVDs include specific diagnostic enzymes, primers, antigens, and antibodies, depending on the test to be performed. These reagents are necessary to refill their test requirements, allowing the equipment to be reused. They can be used alone or in combination with instruments, apparatus, equipment, or systems. Several IVD manufacturers sell high-quality reagents to improve the precision and accuracy of test kits.
Technology Insights
The immunoassay segment held the largest share of the market in 2024. An immunoassay is a biochemical technique that measures the presence or amount of macromolecules or small molecules in a biological sample. Western blot, ELISA, radioimmunoassay, and chemiluminescence immunoassays are some examples of immunodiagnostics. They are widely preferred due to their high specificity and sensitivity. They are comparatively affordable and yield faster results, augmenting the segment’s growth. Additionally, immunoassays enable the analysis of multiple samples simultaneously, reducing the average analytical time.
Application Insights
The infectious diseases segment led the global in vitro diagnostics market in 2024. The rising prevalence of infectious diseases such as HIV, COVID-19, TB, and salmonella fuels the segment’s growth. The World Health Organization (WHO) estimated that around 10.8 million people were diagnosed with TB globally in 2023 caused by Mycobacterium tuberculosis. Infectious diseases are contagious and have a high risk of spreading through the air, necessitating their early detection. The availability of point-of-care diagnostics enables patients and healthcare professionals to detect the presence of infectious diseases from the comfort of their homes, preventing the spread of disease. Technological advancements have led to the development of IVDs that can detect the type of micro-organisms responsible for disease causation.
End-Use Insights
The hospitals segment held a major share of the market in 2024. The segmental growth is attributed to favorable infrastructure and suitable capital investments. This enables hospitals to adopt advanced IVDs for state-of-the-art treatment. Many hospitals have their own laboratories for conducting tests, allowing diagnosing and monitoring patients’ health. The presence of skilled professionals and favorable reimbursement policies encourage patients to prefer hospitals.
Top Companies in the In Vitro Diagnostics Market

BD | F. Hoffmann-La Roche Ltd. | Qiagen | Siemens Healthineers AG | Agilent Technologies, Inc. | Bio-Rad Laboratories, Inc. | QuidelOrtho Corporation | Charles River Laboratories | Abbott | Danaher Corporation | bioMérieux SA | Quest Diagnostics Incorporated | Sysmex Corporation
Recent Breakthroughs
- In August 2024, InBios International, Inc. announced the launch of a new in vitro serological ELISA kit, Strongy Detect IgG ELISA, for the early diagnosis of Strongyloides infection. The kit enables the detection of specific IgG antibodies to Strongyloides recombinant antigens and offers the results within 75 minutes.
- In June 2024, Roche announced the launch of its Ventana Kappa and Lambda Dual ISH mRNA Probe Cocktail assay in countries that accept the EU CE mark. The assay was developed for the detection and differentiation of B-cell lymphomas and plasma cell neoplasms from a normal immune response.
Browse More Insights of Towards Healthcare:
In Vitro Lung Model Market Sales: https://www.towardshealthcare.com/insights/in-vitro-lung-model-market-sizing
Molecular Diagnostics Market Sales: https://www.towardshealthcare.com/insights/molecular-diagnostics-market-sizing
DNA Diagnostics Market Sales: https://www.towardshealthcare.com/insights/dna-diagnostics-market-sizing
Core Clinical Molecular Diagnostics Market Sales: https://www.towardshealthcare.com/insights/core-clinical-molecular-diagnostics-market-sizing
AI in Diagnostics Market Sales: https://www.towardshealthcare.com/insights/ai-in-diagnostics-market-sizing
Stroke Diagnostics and Therapeutics Market Sales: https://www.towardshealthcare.com/insights/stroke-diagnostics-and-therapeutics-market-size
AI in Cancer Diagnostics Market Sales: https://www.towardshealthcare.com/insights/ai-in-cancer-diagnosis-transforming-cancer-care
Oncology Molecular Diagnostics Market Sales: https://www.towardshealthcare.com/insights/oncology-molecular-diagnostics-market-sizing
U.S. Oncology Molecular Diagnostics Market Sales: https://www.towardshealthcare.com/insights/us-oncology-molecular-diagnostics-market-sizing
Biosensors Market Sales: https://www.towardshealthcare.com/insights/biosensors-market-sizing
Segments Covered in the Report
By Product
- Reagents
- Instruments
- Services
By Technology
- Immunoassay
- Instruments
- Reagents
- Services
- Hematology
- Instruments
- Reagents
- Services
- Clinical Chemistry
- Instruments
- Reagents
- Services
- Molecular Diagnostics
- Instruments
- Reagents
- Services
- Coagulation
- Instruments
- Reagents
- Services
- Microbiology
- Instruments
- Reagents
- Services
- Others
- Instruments
- Reagents
- Services
By Application
- Infectious Diseases
- Diabetes
- Oncology
- Cardiology
- Nephrology
- Autoimmune Diseases
- Drug Testing
- Others
By End-Use
- Hospitals
- Home-care
- Laboratory
- Others
By Region
- North America
- US
- Canada
- Asia Pacific
- China
- Japan
- India
- South Korea
- Thailand
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Sweden
- Denmark
- Norway
- Latin America
- Brazil
- Mexico
- Argentina
- Middle East and Africa (MEA)
- South Africa
- UAE
- Saudi Arabia
- Kuwait
For pricing details and customized market report options, click here: https://www.towardshealthcare.com/price/5229
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
Gain access to the latest insights and statistics in the healthcare industry by subscribing to our Annual Membership. Stay updated on healthcare industry segmentation with detailed reports, market trends, and expert analysis tailored to your needs. Stay ahead of the curve with valuable resources and strategic recommendations. Join today to unlock a wealth of knowledge and opportunities in the dynamic world of healthcare: Get a Subscription
Towards Healthcare Recognized by Top Media Outlet
★ Outsourced Pharma: https://www.outsourcedpharma.com/doc/it-s-partly-personal-medicine-in-years-outsourcing-at-billion-0001
About Us
Towards Healthcare is a leading global provider of technological solutions, clinical research services, and advanced analytics to the healthcare sector, committed to forming creative connections that result in actionable insights and creative innovations. We are a global strategy consulting firm that assists business leaders in gaining a competitive edge and accelerating growth. We are a provider of technological solutions, clinical research services, and advanced analytics to the healthcare sector, committed to forming creative connections that result in actionable insights and creative innovations.
Our Trusted Data Partners
Precedence Research | Statifacts | Towards Packaging | Towards Automotive | Towards Food and Beverages | Towards Chemical and Materials | Towards Consumer Goods | Towards Dental | Towards EV Solutions | Nova One Advisor | Healthcare Webwire | Packaging Webwire | Automotive Webwire
For Latest Update Follow Us: https://www.linkedin.com/company/towards-healthcare

Distribution channels: Consumer Goods ...
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release


