
Pulmonary Fibrosis Clinical Trial Pipeline Appears Robust With 110+ Key Pharma Companies Actively Working in the Therapeutics Segment | DelveInsight
The pulmonary fibrosis market is driven by the increasing prevalence of pulmonary fibrosis, especially idiopathic pulmonary fibrosis (IPF), among the aging population, which boosts demand for effective treatments. Innovations in drug development and diagnostics enhance therapy options, while pharmaceutical R&D addresses unmet needs. Favorable reimbursement policies improve access to medications, and greater awareness among stakeholders supports early diagnosis, fueling market growth.
/EIN News/ -- New York, USA, Jan. 28, 2025 (GLOBE NEWSWIRE) -- Pulmonary Fibrosis Clinical Trial Pipeline Appears Robust With 110+ Key Pharma Companies Actively Working in the Therapeutics Segment | DelveInsight
The pulmonary fibrosis market is driven by the increasing prevalence of pulmonary fibrosis, especially idiopathic pulmonary fibrosis (IPF), among the aging population, which boosts demand for effective treatments. Innovations in drug development and diagnostics enhance therapy options, while pharmaceutical R&D addresses unmet needs. Favorable reimbursement policies improve access to medications, and greater awareness among stakeholders supports early diagnosis, fueling market growth.
DelveInsight’s 'Pulmonary Fibrosis Pipeline Insight 2025' report provides comprehensive global coverage of pipeline pulmonary fibrosis therapies in various stages of clinical development, major pharmaceutical companies are working to advance the pipeline space and future growth potential of the pulmonary fibrosis pipeline domain.
Key Takeaways from the Pulmonary Fibrosis Pipeline Report
- DelveInsight’s pulmonary fibrosis pipeline report depicts a robust space with 110+ active players working to develop 120+ pipeline pulmonary fibrosis drugs.
- Key pulmonary fibrosis companies such as Boehringer Ingelheim, Avalyn Pharmaceuticals, Syndax Pharmaceuticals, Endeavor BioMedicines, Humanetics Corporation, Melius Pharma AB, Tvardi Therapeutics, GRI Bio Operations, Daewoong Pharmaceutical, Regend Therapeutics, PureTech Health, Otsuka Holdings, Vicore Pharma, Sunshine Lake Pharma, Bridge Biotherapeutics, InSilico Medicine, Redx Pharma, Trevi Therapeutics, GlaxoSmithKline, Sarepta Therapeutics, Guangdong Raynovent, Lassen Therapeutics, Contineum Therapeutics, and others are evaluating new pulmonary fibrosis drugs to improve the treatment landscape.
- Promising pipeline pulmonary fibrosis therapies such as BI1015550, AP 01, Axatilimab, ENV-101, GKT137831, BIO 300, ME-015, TTI-101, GRI-0621, DWN12088, REGEND001, LYT-100, TAS-115, Buloxibutid, HEC585, BBT-877, ISM001-055, RXC007, Nalbuphine ER, GSK-3915393, ARO-MMP7, ZSP1603, LASN01, PIPE-791, and others are under different phases of Pulmonary Fibrosis clinical trials.
- In January 2025, Mediar Therapeutics announced a global licensing agreement with Eli Lilly and Company to advance MTX-463 into a Phase II clinical trial for idiopathic pulmonary fibrosis (IPF). MTX-463 is a first-in-class human IgG1 antibody designed to neutralize WISP1-mediated fibrotic signaling in several debilitating diseases.
- In October 2024, Trevi Therapeutics company developing the investigational therapy Haduvio (oral nalbuphine ER) for the treatment of chronic cough in idiopathic pulmonary fibrosis (IPF) and refractory chronic cough (RCC), provided updates on its clinical development programs.
- In September 2024, Aileron Therapeutics announced the completion of enrollment in Cohort 2 of the ongoing Phase Ib clinical trial of LTI-03 in IPF patients.
- In July 2024, Bridge Biotherapeutics announced that patient participant enrollment had been completed in the Phase II clinical study of BBT-877, a novel autotoxin (ATX) inhibitor for the treatment of idiopathic pulmonary fibrosis (IPF).
- In July 2024, Huitai BioMedicine (HUITAI) announced that the company's first self-developed and globally patented innovative drug, inhaled HTPEP-001 for the treatment of pulmonary fibrosis, completed the dosing of the first healthy subject in Phase I clinical trial at Beijing Chaoyang Hospital.
- In June 2024, Structure Therapeutics initiated a Phase I clinical trial of LTSE-2578, an oral small molecule antagonist that targets the lysophosphatidic acid 1 receptor (LPA1R) for the treatment of IPF.
- In June 2024, Agomab Therapeutics announced that it had received Orphan Drug Designation from the US Food and Drug Administration (FDA) for AGMB-447, its inhaled, small molecule inhibitor of ALK5. Agomab is evaluating AGMB-447 as a potential treatment for Idiopathic Pulmonary Fibrosis (IPF) in a Phase I clinical trial (NCT06181370).
- In May 2024, Alentis Therapeutics announced that the US Food and Drug Administration (FDA) had granted lixudebart (ALE.F02) Orphan Drug designation for the treatment of Idiopathic Pulmonary Fibrosis (IPF).
- In April 2024, PureTech Health announced that enrollment has been completed in the ELEVATE IPF Phase IIb clinical trial evaluating LYT-100 (deupirfenidone) in patients with idiopathic pulmonary fibrosis (IPF).
- In August 2024, ArkBio CEO Presented Promising IPF Drug AK3280 at 8th Annual IPF Summit in Boston. ArkBio also announced completion of patient enrollment for Phase II study for AK3280.
Request a sample and discover the recent advances in pulmonary fibrosis drugs @ Pulmonary Fibrosis Pipeline Report
The pulmonary fibrosis pipeline report provides detailed profiles of pipeline assets, a comparative analysis of clinical and non-clinical stage pulmonary fibrosis drugs, inactive and dormant assets, a comprehensive assessment of driving and restraining factors, and an assessment of opportunities and risks in the pulmonary fibrosis clinical trial landscape.
Pulmonary Fibrosis Overview
Pulmonary fibrosis is a chronic, progressive lung disorder marked by an excessive build-up of extracellular matrix (ECM) and changes in lung structure. This leads to lung scarring and thickening, impairing gas exchange and causing a gradual decline in lung function. The disease typically presents with symptoms like shortness of breath, chronic cough, and fatigue, with imaging showing typical interstitial fibrosis patterns.
The development of pulmonary fibrosis is driven by complex interactions involving genetic factors, environmental influences, and abnormal cellular responses. A key aspect of pulmonary fibrosis is the disruption of normal wound healing. Under normal circumstances, lung injury triggers repair mechanisms like inflammation, epithelial cell regeneration, and collagen production. In pulmonary fibrosis, however, this repair process becomes chronic and fails to restore normal tissue structure, leading to excessive fibrosis. This is believed to result from an imbalance in pro-fibrotic and anti-fibrotic signals in the lung, with the TGF-β (transforming growth factor-beta) pathway playing a central role. Dysregulation of this pathway promotes persistent fibroblast activation and excessive collagen deposition in the lung tissue.
Pulmonary fibrosis presents with a variety of symptoms, and its progression can vary widely among patients. While the early stages may involve subtle signs, the disease usually worsens over time, leading to significant respiratory and systemic issues. Shortness of breath is one of the earliest symptoms, typically occurring during physical activity, such as climbing stairs or walking uphill. As the disease advances, shortness of breath can occur with minimal exertion or even at rest, as lung scarring reduces the ability to exchange gases effectively.
Non-pharmacological approaches are crucial in managing pulmonary fibrosis, aiming to relieve symptoms, enhance physical function, and preserve quality of life. Preventive measures, such as vaccination against pneumococcus and influenza to prevent respiratory infections, are important. Long-term oxygen therapy is a key non-drug treatment, often used to improve oxygen levels. While it is generally recommended for rest, recent research indicates that using oxygen during physical exertion can also enhance quality of life, though the benefits may be modest.
Find out more about pulmonary fibrosis drugs @ Pulmonary Fibrosis Analysis
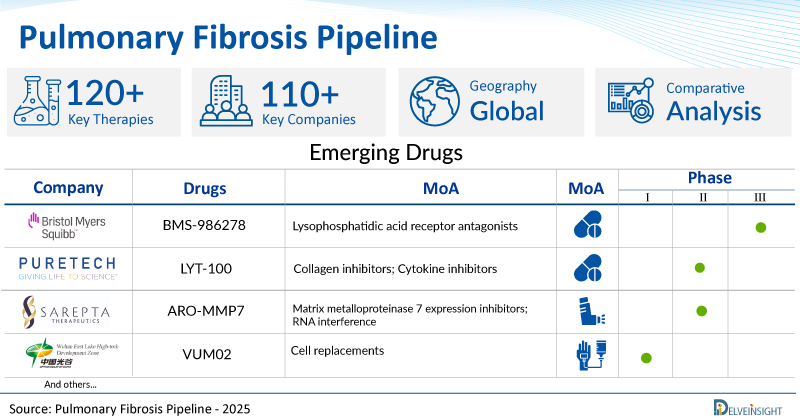
A snapshot of the Pipeline Pulmonary Fibrosis Drugs mentioned in the report:
| Drugs | Company | Phase | MoA | RoA |
| BMS-986278 | Bristol-Myers Squibb | III | Lysophosphatidic acid receptor antagonists | Oral |
| LYT-100 | PureTech Health | II | Collagen inhibitors; Cytokine inhibitors | Oral |
| ARO-MMP7 | Sarepta Therapeutics | I/II | Matrix metalloproteinase 7 expression inhibitors; RNA interference | Inhalation |
| VUM02 | Wuhan Optics Valley Vcanbiopharma Co., Ltd. | I | Cell replacements | Intravenous |
| PMG1015 | Pulmongene Ltd. | I | Immunomodulators | Oral |
Learn more about the emerging pulmonary fibrosis therapies @ Pulmonary Fibrosis Clinical Trials
Pulmonary Fibrosis Therapeutics Assessment
The pulmonary fibrosis pipeline report proffers an integral view of the emerging pulmonary fibrosis therapies segmented by stage, product type, molecule type, route of administration, and mechanism of action.
Scope of the Pulmonary Fibrosis Pipeline Report
- Coverage: Global
- Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
- Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
- Therapeutics Assessment By Route of Administration: Oral, Intravenous, Subcutaneous, Parenteral, Topical
- Therapeutics Assessment By Molecule Type: Recombinant fusion proteins, Small molecule, Monoclonal antibody, Peptide, Polymer, Gene therapy
- Therapeutics Assessment By Mechanism of Action: Lysophosphatidic acid receptor antagonists, Collagen inhibitors, Cytokine inhibitors, Matrix metalloproteinase 7 expression inhibitors, RNA interference, Transforming growth factor beta1 expression inhibitors, Cell replacements
- Key Pulmonary Fibrosis Companies: Boehringer Ingelheim, Avalyn Pharmaceuticals, Syndax Pharmaceuticals, Endeavor BioMedicines, Humanetics Corporation, Melius Pharma AB, Tvardi Therapeutics, GRI Bio Operations, Daewoong Pharmaceutical, Regend Therapeutics, PureTech Health, Otsuka Holdings, Vicore Pharma, Sunshine Lake Pharma, Bridge Biotherapeutics, InSilico Medicine, Redx Pharma, Trevi Therapeutics, GlaxoSmithKline, Sarepta Therapeutics, Guangdong Raynovent, Lassen Therapeutics, Contineum Therapeutics, and others.
- Key Pulmonary Fibrosis Pipeline Therapies: BI1015550, AP 01, Axatilimab, ENV-101, GKT137831, BIO 300, ME-015, TTI-101, GRI-0621, DWN12088, REGEND001, LYT-100, TAS-115, Buloxibutid, HEC585, BBT-877, ISM001-055, RXC007, Nalbuphine ER, GSK-3915393, ARO-MMP7, ZSP1603, LASN01, PIPE-791, and others.
Dive deep into rich insights for new pulmonary fibrosis treatments, visit @ Pulmonary Fibrosis Drugs
Table of Contents
| 1. | Pulmonary Fibrosis Pipeline Report Introduction |
| 2. | Pulmonary Fibrosis Pipeline Report Executive Summary |
| 3. | Pulmonary Fibrosis Pipeline: Overview |
| 4. | Analytical Perspective In-depth Commercial Assessment |
| 5. | Pulmonary Fibrosis Clinical Trial Therapeutics |
| 6. | Pulmonary Fibrosis Pipeline: Late-Stage Products (Pre-registration) |
| 7. | Pulmonary Fibrosis Pipeline: Late-Stage Products (Phase III) |
| 8. | Pulmonary Fibrosis Pipeline: Mid-Stage Products (Phase II) |
| 9. | Pulmonary Fibrosis Pipeline: Early-Stage Products (Phase I) |
| 10. | Pulmonary Fibrosis Pipeline Therapeutics Assessment |
| 11. | Inactive Products in the Pulmonary Fibrosis Pipeline |
| 12. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 13. | Key Companies |
| 14. | Key Products in the Pulmonary Fibrosis Pipeline |
| 15. | Unmet Needs |
| 16. | Market Drivers and Barriers |
| 17. | Future Perspectives and Conclusion |
| 18. | Analyst Views |
| 19. | Appendix |
For further information on the pulmonary fibrosis pipeline therapeutics, reach out @ Pulmonary Fibrosis Therapeutics
Related Reports
Pulmonary Fibrosis Epidemiology Forecast
Pulmonary Fibrosis Epidemiology Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted pulmonary fibrosis epidemiology in the 7MM, i.e., the United States, EU5 (Germany, Spain, Italy, France, and the United Kingdom), and Japan.
Pulmonary Fibrosis Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key pulmonary fibrosis companies, including MediciNova Inc., Jubilant Pharma Limited, Merck & Co. Inc., Horizon Therapeutics Inc., United Therapeutics, Boehringer Ingelheim, Bristol-Myers Squibb Company, Cipla Inc., F. Hoffmann-La Roche Ltd, FibroGen Inc., Avalyn Pharma Inc., CS Pharmaceuticals, among others.
Idiopathic Pulmonary Fibrosis Market
Idiopathic Pulmonary Fibrosis Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key IPF companies, including FibroGen, Hoffmann-La Roche Ltd, United Therapeutics, Boehringer Ingelheim, Pliant Therapeutics, Inc., Galecto Biotech, Horizon Therapeutics, CSL Behring, Kadmon Corporation, LLCs, MediciNova, PureTech, Bristol-Myers Squibb, Nitto Denko Corporation, Vicore Pharma AB, among others.
Idiopathic Pulmonary Fibrosis Pipeline
Idiopathic Pulmonary Fibrosis Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key idiopathic pulmonary fibrosis companies, including FibroGen, United Therapeutics, Bellerophon Therapeutics, MediciNova, Novartis, Endeavor BioMedicines, Pliant Therapeutics, Nitto Denko, Kadmon Pharmaceuticals, Calliditas Therapeutics, Avalyn Pharmaceuticals, PureTech Health, Taiho Pharmaceutical, Bristol-Myers Squibb, Galecto Biotech AB, CSL Behring, Celgene Pharmaceutical, Vicore Pharma, Boehringer Ingelheim, Guangdong Raynovent, Sunshine Lake Pharma co, Suzhou Zelgen Biopharmaceuticals, Algernon Pharmaceuticals, Horizon Therapeutics, Daewoong Pharmaceutical, Metagone Biotech, AstraZeneca, Lung Therapeutics, Bridge Biotherapeutics, AstraZeneca, Kinarus AG, Insmed, Reviva Pharmaceuticals, Annapurna Bio, Guangdong Hengrui Pharmaceutical Co., Ltd, Ark Biosciences, Ocean Biomedical, among others.
Cough in Idiopathic Pulmonary Fibrosis Market
Cough in Idiopathic Pulmonary Fibrosis Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key cough in IPF companies, including NeRRe Therapeutics, Trevi Therapeutics, Algernon Pharmaceuticals, Seyltx Inc., Melius Pharma AB, Cellular Sciences, Emphycorp, among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences.
Connect with us at LinkedIn

Contact Us
Shruti Thakur
info@delveinsight.com
+14699457679
www.delveinsight.com
Distribution channels: Banking, Finance & Investment Industry, Healthcare & Pharmaceuticals Industry, Media, Advertising & PR, Science ...
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release

